Cellular Microbiology (Schubert-Unkmeir lab)
Neisseria meningitidis (Nm, meningococcus) is an obligate commensal in humans, colonizing the nasopharyngeal mucosa usually without affecting the host. After the onset of colonization, Nm strains occasionally penetrate the mucosal membrane and enter the bloodstream to cause severe septicemia. Following bacteremia, Nm may bind and subsequently cross the meningeal blood-cerebrospinal fluid barrier (BCSFB) to enter the subarachnoid space and cause meningitis.
BCSFB penetration and development of novel multicellular in vitro models
The group’s major research focus lies on molecular interactions of Nm with microvascular brain endothelial cells (BECs) and mechanisms of BCSFB penetration. For this purpose, we use a variety of tissue culture techniques and cellular models, as well as a wide spectrum of innovative molecular, biochemical methods and imaging techniques. One current research topic covers the development of human, multicellular models of the BCSFB, which includes stem cell-derived BECs and leptomeningeal cells.
Sphingolipids and meningococcal meningitis
Sphingolipid-enriched membrane microdomains contribute to a variety of cellular processes, including signal transduction and vesicle trafficking, and can be exploited by pathogens such as Nm for host cell entry. Current work in this area is focused on the role of the downstream metabolite sphingosine 1-phosphate (S1P) and S1P-producing Sphingosine kinases 1 and 2 (Sphk1/2).
Nasopharyngeal colonization and transmigration
Meningococcal colonization of the nasopharynx is a dynamic process. Nm must mediate complex interactions with host epithelium, and persist in the presence of normal microflora and host mucosal defenses. We are currently developing an air-liquid Interface (ALI) human epithelial model to study the impact of Nm on the architecture and physiology of polarized epithelium and aim to characterize the process of meningococcal penetration of this barrier.
Immunopathogenesis of Post-Infectious Lyme Arthritis in Synovial Cultures
This newly funded project, supported by the Interdisciplinary Center for Clinical Research (IZKF), focuses on the immune mechanisms underlying antibiotic-refractory Lyme arthritis (ARLA)—a chronic inflammatory condition that persists despite appropriate antibiotic therapy. While ARLA in North America is primarily associated with B. burgdorferi sensu stricto and a dysregulated immune response against OspA, preliminary data suggest that distinct Borrelia species and antigens may drive ARLA in Europe.
In collaboration with PD Dr. Henner Morbach (Department of Pediatrics), the project aims to 1) identify immunodominant Borrelia antigens linked to European ARLA, 2) characterize the proinflammatory potential of various European Borrelia species, and 3) establish a 3D synovial tissue model to study pathogen-host interactions and T cell differentiation.
In the long term, the project seeks to identify high-risk Borrelia strains and early serological markers of immune dysregulation, paving the way for risk-adapted and personalized treatment strategies.
December 2025 | International hands-on workshop at the Indian Institute of Technology (IIT) Indore
As part of the SPARC-GIANTS program (Scheme for Promotion of Academic and Research Collaboration – German Indian Academic Network for Tomorrow), funded by the DAAD (German Academic Exchange Service), two of our doctoral researchers, Alina Weinmann and Johannes Stumpf, were selected to participate in a one-week international hands-on workshop on integrated biomedical devices.
Together with eight other students from various institutes of JMU and UKW in Würzburg, the two doctoral researchers traveled to Indore, a city of around two million inhabitants in central India. The workshop took place at the Indian Institute of Technology (IIT) Indore, one of the leading research and higher-education institutions in India. The program was coordinated by Prof. Dr. Srikanth Karnati (Institute of Anatomy and Cell Biology, JMU Würzburg).
The scientific program covered a wide range of topics, including biomedical ultrasound simulation and experimental data acquisition, light-guided biofabrication, and high-resolution electrophysiology. Participants had the opportunity to gain hands-on experience and further develop their research expertise in the field of biomedical device technology. A key focus of the workshop was the scientific exchange with the Indian students and the establishment of international networks.
In addition to the scientific activities, there was also time to gain cultural insights, for example during a trip to the historic ruins of Mandu.

Januar 2025 | Successful Participation in the SLBiol Young Researchers Contest

We are delighted to announce that our doctoral candidate, Alina Weinmann, participated in the prestigious SLBiol Young Researchers Contest and emerged as the winner!
This achievement includes full coverage of congress fees, and accommodation by the organizers. Alina will represent our institution at the 13th International Ceramide Conference, a FEBS Special Meeting, taking place from May 25–30, 2025, in Varna, Bulgaria. The conference will highlight the latest advancements in sphingolipid biology.
Congratulations to Alina on this outstanding success!
https://sphingolipidbiology2025.febsevents.org/
Juli 2024 | Defended PhD thesis
On 09.07.2024, Ingo Fohmann successfully defended his PhD thesis entitled “The Role of Sphingosine 1-phosphate and S1PR1-3 in the Pathophysiology of Meningococcal Meningitis” in front of a public audience and his Thesis Committee consisting of Prof. Alexandra Schubert-Unkmeir, Prof. Christian Stigloher and Prof. Jürgen Seibel as well as Prof. Emanuel Saliba, who kindly chaired the defense.
Juli 2024 | Untersuchungen zur Rolle von Mukus während der Interaktion von N. meningitidis mit respiratorischen Zellen

Katherina Mohort hat kürzlich ihre neuesten Ergebnisse zur Untersuchung der Interaktion von Krankheits- und Trägerisolaten von N. meningitidis im Hinblick auf ihre Fähigkeit an Calu-3-Zellen, die als ALI-Zellkulturmodell kultiviert wurden, veröffentlicht.
Die ersten Ergebnisse zeigten, dass die Krankheitsisolate durchweg invasiver waren und die Epithelbarriere besser überwinden konnten als die Trägerisolate.
Mit Hilfe von TEER (transepithelial electrical resistance) Messungen und Permeabilitätstests konnte sie zeigen, dass die Barriere nach 24 Stunden Infektion intakt bleibt. Durch die Exposition der Luft produzieren Calu-3 Zellen eine dicke Mukusschicht, die eine erste Verteidigungslinie gegen Krankheitserreger der Atemwege darstellt.
Die Analyse der Interaktion der Meningokokken mit dem Mukus zeigte, dass alle Isolate in der äußeren Mukusschicht gefangen waren und nur bis zu 10 % direkt mit den Zellen interagieren konnten.
Diese Ergebnisse konnten kürzlich mit Katherina Mohort als geteilte Erstautorenschaft in Frontiers Cellular and Infection Microbiology veröffentlicht werden.
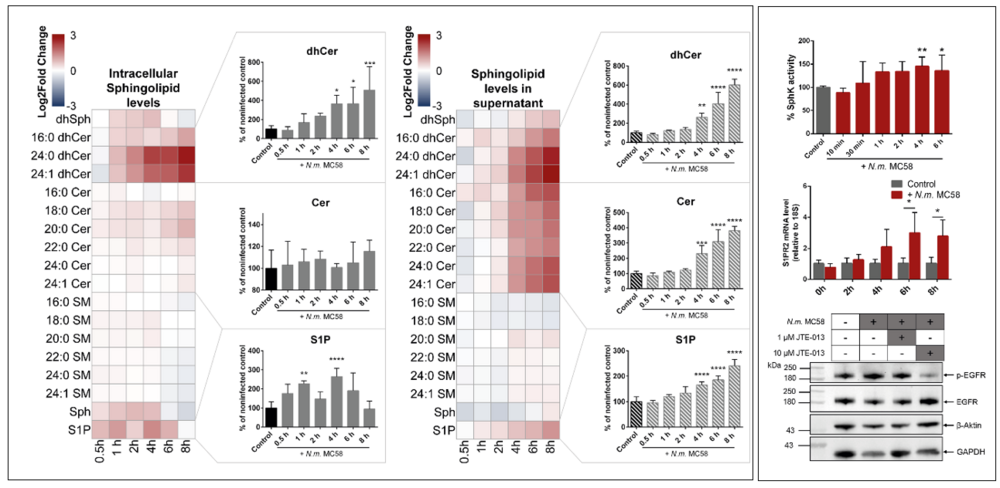
Januar 2024 | Meningococci manipulate sphingolipid signaling at the blood-brain barrier
Sphingolipid-enriched microdomains are contributing to various cellular processes, including signal transduction and vesicular transport. Additionally, they can be exploited by pathogens like Neisseria meningitidis to enter host cells. In the paper recently published in PLOS Pathogens, Ingo Fohmann (in the lab of Prof. Schubert-Unkmeir) could show that Neisseria meningitidis induces the release of S1P from brain endothelial cells to favor uptake through the activation of plasma membrane located S1P receptor 2. These results indicate a new uptake mechanism of Neisseria meningitidis and therefore hind towards novel targets for adjuvant therapy using S1P receptor modulators during meningococcal infection.
The research was supported by the DFG-funded research training group GRK2581 in cooperation with colleagues from Freie Universität Berlin (Agata Prell, Dr. Schumacher and Prof. Kleuser, Institute for Pharmazie) and Virginia Commonwealth University (Dr. Weigel and Prof. Dr. Spiegel). Prof. Spiegel discovered S1P in 1991 as bioactive lipid involved in cell proliferation.
October 8-13, 2023 | FEBS Special Meeting on Sphingolipid Biology
Ingo Fohmann presented his work titled “Neisseria meningitidis manipulates the SphK-S1P-S1PR axis to invade the brain endothelium” as a poster and a flash talk during the FEBS Special Meeting on Sphingolipid Biology, which took place in Funchal, Portugal during October 8-13 2023. He came back from this stimulating conference with many new ideas and exciting technical and mechanistic insights into field of Sphingolipids. Congratulations to Ingo for receiving the FEBS YTF travel grant, which covered his attendance fee for the conference.
September 24-29, 2023 | 23rd International Pathogenic Neisseria Conference (IPNC)
Our group was represented with two contributions at this year's IPNC conference (‘Neisseria meningitidis exploits SphK-S1P-S1PR2 axis for EGFR activation and invasion in brain endothelial cells’ data from Ingo and ‘Development of a multicellular in vitro model of the meningeal blood-CSF barrier to study Neisseria meningitidis infection’ data from Leo). Topics on host-pathogen interaction were discussed, as well as therapeutic/antimicrobial resistance/diagnostics, metatranscriptomic analysis, epidemiology and clinical diseases, including invasive meningococcal disease trends in South Africa, meningococcal eye infections and longitudinal meningococcal carriage in adolescents and young adults in South Australia. A number of great keynote speeches were given, among them ‘25 years of MLST’ given by Martin C.J. Maiden.
#IPNC2023
Scientific exchange between AG Schubert-Unkmeir and Research group Prof. Wells
Scientific exchange with Tiantong Zhao from the research group of Prof. Jerry Wells, Chair Host Microbe Interactomics, Wageningen University and Research –
Both groups have a major research interests finding out how bacteria cross the blood-brain/blood-cerebrospinal fluid barrier.
Picture: Left Fatemeh Nosratabadi (PhD student research group Prof. A. Schubert-Unkmeir) right Tiantong Zhao (PhD student research group Prof. Jerry Wells).
Entwicklung neuartiger multizellulärer Modelle für die Meningokokkenforschung
Leo Endres hat seine neuesten Ergebnisse über die Entwicklung neuartiger mutlizellulärer in vitro Modelle der Blut-Liquor Schranke zur Untersuchung von Neisseria meningitidis Infektionsprozessen publiziert. Hierfür wurden spezielle Hirnendothelzellen aus induziert pluripotenten Stammzellen differenziert und auf einer porösen Membran mit Leptomeningealzellen kultiviert. Diese neuen Modelle zeichnen sich durch ihre erhöhte Vergleichbarkeit mit der menschlichen Physiologie aus und eignen sich im Infektionskontext besonders, um pathogene Veränderung und Überwindung der Blut-Liquor-Barriere zu untersuchen. In dieser Studie wurde festgestellt, dass Meningokokken Infektion zu verringerter Expression von Zellverbindungsproteinen im Hirnendothel führt und die allgemeine Barrierefunktion schädigt. Außerdem zeigten sich Indizien, die für eine Kombination aus trans- und parazellulärer Transmigration der Bakterien durch die Barriere sprechen.
September 2022: Participation at DGHM Congress

Simon Peters presented his current work on the “Impact of glycosphingolipids on meningococcal pathogenicity in an Air-Liquid-Interface model of the nasopharyngeal epithelial barrier” during this year’s DGHM (German Society for Hygiene and Microbiology) Congress, which has taken place in Berlin from 05.09. – 07.09.2022.
July 2022: Poster prize at the Gordon Research Conference

Congratulations to Leo Endres, who received an award for his poster at the prestigious Gordon Research Conference ‘Barriers of the CNS’, which took place in New Hampshire, USA, from June 12-17. Poster prizes of up to $1000 were awarded to 6 out of the 110 posters presented at the conference. On his poster, Leo presented data from his project “Development of a multicellular in vitro model of the meningeal blood-CSF barrier to study Neisseria meningitidis infection”, recently submitted for publication.
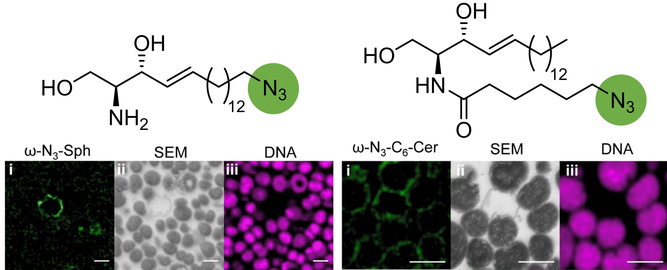
‘Click-AT-CLEM’ for imaging and tracking azido-functionalized sphingolipids in bacteria

In a collaboration project between researchers from IHM (AG Schubert-Unkmeir) together with the groups of Prof. J. Seibel, Prof. M. Sauer, Prof. C. Stigloher and Prof. B. Kleuser, introduce ‘click-AT-CLEM’, a labeling technique for correlated light and electron microscopy (CLEM) based on the super-resolution array tomography (srAT) approach and bio-orthogonal click chemistry for imaging of azido-tagged sphingolipids in Neisseria meningitidis at subcellular level. Click-AT-CLEM imaging and mass spectrometry clearly revealed efficient incorporation of azido-tagged sphingolipids into the outer membrane of the Gram-negative bacterium as an underlying cause of their antimicrobial activity.
Reference: Peters, S., Kaiser, L., Fink, J. Schumacher, F., Perschin, V., Schlegel, J. , Sauer, M., Stigloher, C., Kleuser, B., Seibel, J. and Schubert-Unkmeir, A. Click-correlative light and electron microscopy (click-AT-CLEM) for imaging and tracking azido-functionalized sphingolipids in bacteria. Sci Rep 11, 4300 (2021). https://doi.org/10.1038/s41598-021-83813-w
Funding FOR2123/DFG and GRK2581/DFG
State of the art:
Interaction with brain endothelial cells (BECs) is central to the pathogenicity of Neisseria meningitidis infections. N. meningitidis is able to modulate sphingolipid content on BECs and uses ceramide-enriched membrane platforms (CRPs) as an entry portal into these cells. We have shown that the formation of CRP is the result of transient activation of acid sphingomyelinase by the bacteria, followed by the release of ceramide on BECs. Ceramide can further be metabolized to sphingosine-1-phosphate (S1P), a bioactive lipid mediator that regulates many processes in development, physiology and pathology. Signal transduction via S1P and its associated S1P receptors plays an important role in the function and integrity of the blood-brain barrier (BBB).
Previous work:
In the first funding period of the RTG 2581 the quantitative measurement of a variety of molecular species of sphingolipids in BECs infected with N. meningitidis was determined using LC-MS/MS. The data analysis revealed a significant time-dependent increase of S1P release into the culture supernatant. Elevated S1P could be attributed to a transient increase in the expression and activity of the S1P-synthesising enzyme sphingosine kinase 1 (Sphk1), while the expression of the S1P transporter Spns2 was not changed. Furthermore, the expression of further S1P-related genes (Sphk2, S1P phosphatase 1/2 (SGPP1/2), S1P lyase (SGPL1)) was not altered. Treatment of cells with pilus-enriched supernatants (sheared fractions) resulted in a significant increase of SphK activity, demonstrating that the type IV pilus (TFP) of N. meningitidis contributes to SphK activity, which was further verified by blocking the interaction of TFP with its receptor CD147 [1]. S1P exerts its functions by binding to its specific receptors, of which three, S1P1, S1P2 and S1P3, are commonly expressed on BECs [2]. We found that infection with N. meningitidis increases the expression of S1P2, whereas expression of S1P1 or S1P3 is not affected. We have previously shown that N. meningitidis causes epidermal growth factor receptor (EGFR) activation in BECs to induce cytoskeletal rearrangements necessary for uptake [3]. Interestingly, EGFR phosphorylation could be significantly inhibited by the S1P2 receptor antagonist JTE-013. In parallel, infection in the presence of JTE-013 also blocked N. meningitidis uptake by BECs, demonstrating that N. meningitidis exploits the S1P-S1P2 axis for EGFR activation and thus invasion.
Major goal and working plan:
S1P has been shown to play an important role in regulating BBB integrity by mediating signals via receptor isoforms S1P1-3 on BECs. We will analyze whether S1P1-3 receptor-modulating drugs will have a beneficial effect on the impaired integrity of the BBB observed in response to N. meningitidis infection (Aim1). Furthermore, we want to investigate whether the SphK/S1P/S1PR2 pathway contributes to the recruitment of ezrin during N. meningitidis infection and thus is involved in the formation of protrusions and bacterial internalization (Aim2). We will also determine the role of increased dhCer levels regarding changes of the biophysical properties of the plasma membrane (Aim3).
References:
1. Bernard SC, Simpson N, Join-Lambert O, Federici C, Laran-Chich MP, Maissa N, et al. Pathogenic Neisseria meningitidis utilizes CD147 for vascular colonization. Nature medicine. 2014;20(7):725-31. Epub 2014/06/02. doi: 10.1038/nm.3563. PubMed PMID: 24880614.
2. Prager B, Spampinato SF, Ransohoff RM. Sphingosine 1-phosphate signaling at the blood-brain barrier. Trends Mol Med. 2015;21(6):354-63. Epub 2015/05/06. doi: 10.1016/j.molmed.2015.03.006. PubMed PMID: 25939882.
3. Slanina H, Mundlein S, Hebling S, Schubert-Unkmeir A. Role of Epidermal Growth Factor Receptor Signaling in the Interaction of Neisseria meningitidis with Endothelial Cells. Infection and immunity. 2014;82(3):1243-55. Epub 2014/01/01. doi: 10.1128/iai.01346-13. PubMed PMID: 24379285.
State of the art:
N. meningitidis (the meningococcus) colonizes the nasopharynx mainly as a commensal, being carried asymptomatically by 5–10% of the healthy population in non-endemic times. In rare cases, N. meningitidis can cross the epithelium of the nasopharynx, gain access to the bloodstream and cause severe septicemia and/or meningitis. One of the main factors affecting the pathogenicity of N. meningitidis is the ability to penetrate the vascular endothelial cell layer of the blood-cerebrospinal fluid barrier (B-CSFB) and infect the meninges. Although the role of adhesins and invasins in the virulence of N. meningitidis has been demonstrated, the mechanisms that govern meningococcal penetration of brain endothelial cells (BECs) and subsequent interaction with leptomeningeal cells are still not fully understood.
Previous work:
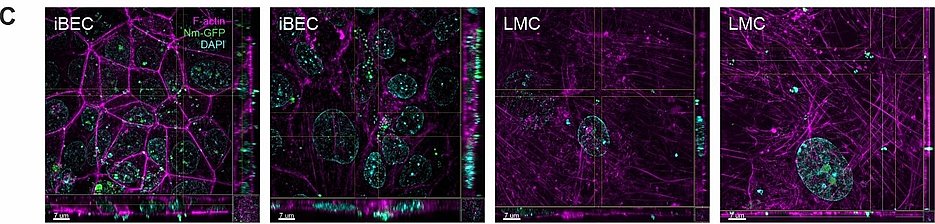
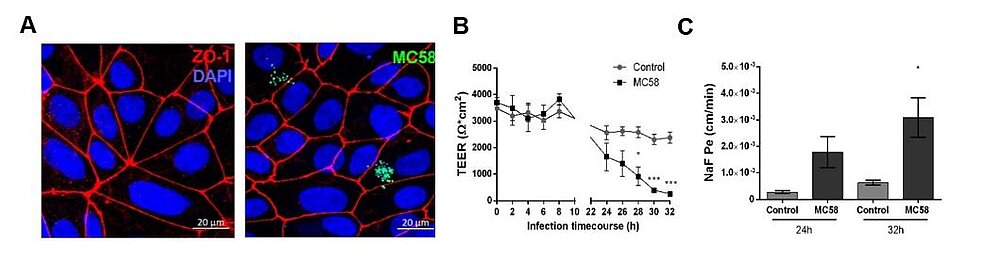
In the first funding period of the GRK 2157 induced pluripotent stem cells (iPSC)-derived BEC like cells have been implemented as a novel cellular model for N. meningitidis infection in close collaboration with Dr. Antje Appelt-Menzel/PD Dr. M. Metzger (LS TERM Würzburg) and Dr. Brandon Kim (University of Alabama)/Prof. E. Shusta (University of Wisconsin) according to previously described methods [1-5]. By using gentamicin protection and immunofluorescence assays we confirmed that multiple N. meningitidis wildtype strains and mutants followed similar phenotypes to previously described models (Fig.1). Recruitment of the recently published pilus adhesion receptor CD147 underneath meningococcal microcolonies in iPSC-BECs was demonstrated. N. meningitidis was found to directly disrupt the three tight junction proteins ZO-1, Occludin, and Claudin-5 at late time points of infection, which became frayed and/or discontinuous upon infection. This destruction was preceded by, and might be dependent on, SNAI1 activation (a transcriptional repressor of tight junction proteins). In accordance with tight junction loss, a sharp loss in TEER, and an increase in sodium fluorescein (NaF) permeability could be shown (Fig.1). Notably, bacterial transmigration correlated with junctional disruption, indicating that a paracellular route contributes for bacterial crossing of BECs. In addition, RNA-Seq data analyses of sorted, infected iPSC-BECs were established in collaboration with A. Westermann (HIRI Wuerzburg) providing expression data of N. meningitidis-responsive host genes not previously described thus far to play a role in N. meningitidis infection of BECs [6]. However, while iPSC-BECs formed a reasonable barrier with high TEER values, these cells lack significant cytokine/chemokine secretion after challenge with N. meningitidis [6]. Since the inflammatory response plays a primordial role in the pathogenesis of meningococcal meningitis, we will continue using both, iPSC-derived BEC-like cells as well as hCMEC/D3 cells, that have been established as state of the art in vitro model in recent years for N. meningitidis infection of the B-CSFB.
Working hypothesis and work plan:
The B-CSFB is composed of brain ECs surrounded by leptomeningeal cells. In the second application period we now use the current model and developed a human B-CSFB in vitro co-culture transfer model. The co-culture model is established consisting of a confluent layer of BECs (iPSC-derived BEC-like cells and hCMEC/D3) on the apical side and a monolayer of leptomeningeal cells on the basolateral side of a microporous membrane for use in mechanistic studies on bacterial transmigration, permeability changes (monitored by NaF transport) and TEER values. Leptomeningeal cells have already been received from our collaboration partner, Prof. M. Christodoulides (University of Southampton) and successfully implemented.
We currently characterize and monitor the quality of the in vitro human B-CSFB co-culture model using transmission electron microscopy (TEM) in collaboration with C. Stigloher (Imaging Core Facility Biozentrum, Würzburg). Sequential events of bacterial adhesion, cellular uptake, localisation and transcytosis are analysed in detail by TEM imaging as well as super-resolution microscopy (SIM and dSTORM/PALM) in collaboration with M. Sauer (Biozentrum, Würzburg).
RNA-Seq data analyses of sorted, infected BECs provided differential regulation of genes involved in hypoxia [6]. Hypoxia is a common feature during inflammation associated with bacterial infection, however the role of hypoxia on N. meningitidis B-CSFB infection has not been elucidated so far. Since activation of hypoxia-inducible factor 1 (HIF-1) transcription factor is the most recognized pathway adopted by hypoxic cells we will determine activation of HIF-1 in N. meningitidis infected BECs and analyse the role of this pathway in maintaining/disturbing B-CSFB function. The contribution of bacterial components (LOS, Pili, NadA) directly involved in HIF-1 activation by the pathogen will be addressed. The different strategies (BECs/leptomeningeal co-cultures, dynamic exposure) are very promising to improve the current in vitro models and will help to reveal useful sub-cellular details to provide evidence for the mechanism of bacterial transmigration in the human B-CSFB model.
References:
1. Appelt-Menzel A, Cubukova A, Gunther K, Edenhofer F, Piontek J, Krause G, et al. Establishment of a Human Blood-Brain Barrier Co-culture Model Mimicking the Neurovascular Unit Using Induced Pluri- and Multipotent Stem Cells. Stem cell reports. 2017;8(4):894-906. Epub 2017/03/28. doi: 10.1016/j.stemcr.2017.02.021. PubMed PMID: 28344002; PubMed Central PMCID: PMCPMC5390136.
2. Lippmann ES, Azarin SM, Kay JE, Nessler RA, Wilson HK, Al-Ahmad A, et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature biotechnology. 2012;30(8):783-91. Epub 2012/06/26. doi: 10.1038/nbt.2247. PubMed PMID: 22729031; PubMed Central PMCID: PMCPMC3467331.
3. Endres LM, Schubert-Unkmeir A, Kim BJ. Neisseria meningitidis Infection of Induced Pluripotent Stem-Cell Derived Brain Endothelial Cells. Journal of visualized experiments : JoVE. 2020;(161). Epub 2020/08/04. doi: 10.3791/61400. PubMed PMID: 32744533.
4. Kim BJ, Schubert-Unkmeir A. In Vitro Models for Studying the Interaction of Neisseria meningitidis with Human Brain Endothelial Cells. Methods in molecular biology (Clifton, NJ). 2019;1969:135-48. Epub 2019/03/17. doi: 10.1007/978-1-4939-9202-7_10. PubMed PMID: 30877675.
5. Doran KS, Fulde M, Gratz N, Kim BJ, Nau R, Prasadarao N, et al. Host-pathogen interactions in bacterial meningitis. Acta neuropathologica. 2016;131(2):185-209. Epub 2016/01/09. doi: 10.1007/s00401-015-1531-z. PubMed PMID: 26744349; PubMed Central PMCID: PMCPMC4713723.
6. Martins Gomes SF, Westermann AJ, Sauerwein T, Hertlein T, Forstner KU, Ohlsen K, et al. Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells as a Cellular Model to Study Neisseria meningitidis Infection. Frontiers in microbiology. 2019;10:1181. Epub 2019/06/14. doi: 10.3389/fmicb.2019.01181. PubMed PMID: 31191497; PubMed Central PMCID: PMCPMC6548865.
Dr. rer. nat. Ingo Fohmann
Present address:
Boston Children's Hospital
Mail: ingo.fohmann(at)childrens.harvard.edu
Mail: ingo.fohmann(at)uni-wuerzburg.de


Fatemeh Nosratabadi
PhD student
Tel.: +49 931 31-84472
Mail: fatemeh.nosratabadi(at)uni-wuerzburg.de

Prof. Dr. med. Alexandra Schubert-Unkmeir
Group leader
Tel.: +49 931 31-46721
Mail: alexandra.schubert-unkmeir(at)uni-wuerzburg.de



Fohmann I, Weinmann A, Schumacher F, Peters S, Prell A, Weigel C, Spiegel S, Kleuser B, Schubert-Unkmeir A. Sphingosine kinase 1/S1P receptor signaling axis is essential for cellular uptake of Neisseria meningitidis in brain endothelial cells. PLOS Pathogens . 2023;19(11):e1011842. doi: 10.1371/journal.ppat.1011842. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10715668/
Endres LM, Jungblut M, Divyapicigil M, Sauer M, Stigloher C, Christodoulides M, Kim BJ, Schubert-Unkmeir A. Development of a multicellular in vitro model of the meningeal blood-CSF barrier to study Neisseria meningitidis infection. Fluids Barriers CNS. 2022 Oct 26;19(1):81. doi: 10.1186/s12987-022-00379-z. PMID: 36289516; PMCID: PMC9597984. https://pubmed.ncbi.nlm.nih.gov/36289516/
Peters S, Fohmann I, Rudel T and Schubert-Unkmeir A, A comprehensive review on the interplay between Neisseria spp. and host sphingolipid metabolites, cells. Cells. 2021 Nov 17;10(11):3201. doi: 10.3390/cells10113201.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8623382/
Krone M, Gütling J, Wagener J, Lâm TT, Schoen C, Vogel U, Stich A, Wedekink F, Wischhusen J, Kerkau T, Beyersdorf N, Klingler S, Backes S, Dölken L, Gasteiger G, Kurzai O, Schubert-Unkmeir A (2021) Performance of Three SARS-CoV-2 Immunoassays, Three Rapid Lateral Flow Tests, and a Novel Bead-Based Affinity Surrogate Test for the Detection of SARS-CoV-2 Antibodies in Human Serum. J Clin Microbiol 59(8):e0031921.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8373215/
Peters S, Kaiser L, Fink J, Schumacher F, Perschin V, Schlegel J, Sauer M, Stigloher C, Kleuser B, Seibel J, Schubert-Unkmeir A. (2021) Click-correlative light and electron microscopy (click-AT-CLEM) for imaging and tracking azido-functionalized sphingolipids in bacteria. Sci Rep. 2021 Feb 22;11(1):4300. doi: 10.1038/s41598-021-83813-w.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7900124/
Schlegel J, Peters S, Doose S, Schubert-Unkmeir A, Sauer M (2019) Super-resolution microscopy reveals local accumulation of plasma membrane gangliosides at Neisseria meningitidis invasion sites. Front Cell Dev Biol. 2019 Sept 7, 194.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6753371/
Martins Gomes SF, Westermann AJ, Sauerwein T, Hertlein T, Förstner KU, Ohlsen K, Metzger M, Shusta EV, Kim BJ, Appelt-Menzel A, Schubert-Unkmeir A. Induced Pluripotent Stem Cell-Derived Brain Endothelial Cells as a Cellular Model to Study Neisseria meningitidis Infection. Front Microbiol. 2019 May 29;10:1181
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6548865/
Peters S, Schlegel J, Becam J, Avota E, Sauer M, Schubert-Unkmeir A. Neisseria meningitidis type IV pili trigger Ca2+-dependent lysosomal trafficking of the acid sphingomyelinase to enhance surface ceramide levels. Infect Immun. 2019 Jun 3. pii: IAI.00410-19. doi: 10.1128/IAI.00410-19.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6652772/
Herrmann JB, Muenstermann M, Strobel L, Schubert-Unkmeir A, Woodruff TM, Gray-Owen SD, Klos A, Johswich KO. Complement C5a Receptor 1 Exacerbates the Pathophysiology of N. meningitidis Sepsis and Is a Potential Target for Disease Treatment. MBio. 2018 Jan 23;9(1). pii: e01755-17. doi: 10.1128/mBio.01755-17.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5784250/
Becam J, Walter T, Burgert A, Schlegel J, Sauer M, Seibel J, Schubert-Unkmeir A. Antibacterial activity of ceramide and ceramide analogs against pathogenic Neisseria. Sci Rep. 2017 Dec 15;7(1):17627.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5732201/
Burgert A, Schlegel J, Bécam J, Doose S, Bieberich E, Schubert-Unkmeir A, Sauer M. Characterization of Plasma Membrane Ceramides by Super-Resolution Microscopy. Angew Chem Int Ed Engl. 2017 Apr 5. doi: 10.1002/anie.201700570.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5549273/
von Papen M, Oosthuysen WF, Becam J, Claus H, Schubert-Unkmeir A. Disease and Carrier Isolates of Neisseria meningitidis Cause G1 Cell Cycle Arrest in Human Epithelial Cells. Infect Immun. 2016 Sep 19;84(10):2758-70.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5038092/
Oosthuysen WF, Mueller T, Dittrich MT, Schubert-Unkmeir A. Neisseria meningitidis causes cell cycle arrest of human brain microvascular endothelial cells at S phase via p21 and cyclin G2. Cell Microbiol. 2016 Jan;18(1):46-65.
https://pubmed.ncbi.nlm.nih.gov/26149128/
Simonis, A., Hebling, S., Gulbins, E., Schneider-Schaulies, S. and Schubert-Unkmeir, A. Differential activation of acid sphingomyelinase and ceramide release determines invasiveness of Neisseria meningitidis into brain endothelial cells. PLoS Pathog. 2014 Jun 12;10(6):e1004160.
https://pubmed.ncbi.nlm.nih.gov/24945304/
Further publications of the research group on Pubmed